Abstract
Introduction: Although most patients (pts) with cutaneous T-cell lymphoma (CTCL) have an indolent course, outcomes for advanced stages (≥IIB) are suboptimal (Dummer R, et al. Nat Rev Dis Primers, 2021). A more aggressive approach of combination cytotoxic therapies in CTCL in the past has been shown to result in higher response rates, but sequential therapies have similar disease-free and overall survival and less toxicities (Kaye FS et al, NEJM, 1989). With the advent of several biological agents in CTCL with favorable toxicity profiles compared to cytotoxics, including the CD30-directed antibody-drug conjugate brentuximab vedotin (BV), and HDAC inhibitors (HDACi), rational novel combinatorial approaches may be beneficial as response rates for single agents remain modest. Evidence suggests that HDAC inhibition may upregulate CD30 expression (Hasanali ZS et al, Sci Transl Med, 2015), supporting the combination of the HDACi romidepsin (R) with BV in pts with CTCL.
Methods: In this multicenter phase I trial of R+BV, pts age ≥18 with stage ≥IB CTCL, good organ function, ECOG PS≤2, <Grade (G) 2 neuropathy, who required systemic treatment were enrolled irrespective of CD30 expression. A traditional "3+3" design with 4 dose levels (DL -2, -1, 0, & 1) was used to define the maximum tolerated dose (MTD). Prior HDACi or BV use was allowed. Enrollment started at DL 0, with R given at 10mg/m2 on days (D) 1, 8 & 15, and BV at 1.2mg/kg (max. 120mg) on D1 & 15 of a 28 day cycle for ≤16 cycles. At DL 1, the R dose was 14mg/m2, with BV 1.2mg/kg, both on D1 & 15. Dosing in the de-escalation cohorts -1 and -2 was as follows: R 10mg/m2 & BV 1.2mg/kg D1 & 15 (DL-1) or R 10mg/m2 D1, 8 & 15 with BV 0.9mg/kg D1 &15 (DL-2). Once the MTD was established, 9 additional pts were enrolled in an expansion cohort. Response was measured using the Global Response Score (GRS; Olsen EA et al. JCO, 2011) using the mSWAT for skin assessment, and flow cytometry and CT imaging every 3 cycles for assessment of extracutaneous sites. There was a "run-in" phase of treatment with R alone given 14 days prior to D1 (D-14) with skin biopsies taken at baseline and prior to C1D1 to assess changes in CD30 expression. The trial was registered in clinicaltrials.gov (NCT02616965).
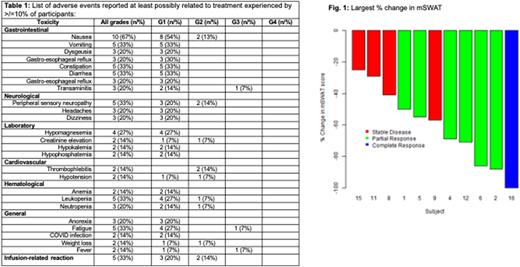
Results: A total of 15 pts were enrolled (DL0: n=3; DL1: n=3; expansion cohort: n=9). No pts were enrolled in the de-escalation cohorts. No pt in the dose finding cohorts experienced a DLT, and DL1 was determined as the MTD. In the expansion cohort at DL1, 9 pts were enrolled, 1 of which experienced a DLT (G3 fatigue >72h) during cycle 1. Median age was 65 years (range 51-79); 83% (n=12) were male; median ECOG PS 1 (0-2); median prior lines of systemic therapy 2 (0-4), including 2 pts with prior HDACi, and 3 pts with prior BV exposure. All pts had Mycosis fungoides (MF) or Sezary syndrome (SS). Stage at enrollment was stage IB in 2 (13%), IIB in 7 (47%), IVA1 and IVB in 1 each (7% each) and IVA2 in 4 (27%). The most common treatment-related adverse events (AE) were nausea (67%), vomiting, constipation, diarrhea, fatigue, leukopenia, peripheral sensory neuropathy (33% each), gastro-esophageal reflux, dizziness, dysgeusia, transaminitis, headaches, and neutropenia (20% each; see Table 1). No pts experienced ≥G4 AE. The only G3 AEs observed were transaminitis, fatigue and fever (n=1 each), all resolved spontaneously. Response assessment at data cut off on 7/1/22 was available for 11 of 15 pts. The overall response rate (ORR) by GRS was 64% (7/11; 95%CI 35-92%; n=1 CR). Both pts with prior HDACi exposure, and 2 of 3 with prior BV exposure responded. The median change in mSWAT was a decrease of 57% from baseline (range -25 to -100%, Fig 1). At data-cut off, 1 pt remained on treatment, while 7 pts (47%) came off treatment due to progression, 2 (13%) each due to non-adherence related to COVID-19 concerns and toxicities (recurrent thrombophlebitis; fatigue), and 1 (7%) each due to patient preference, provider preference and completion of study treatment after 16 cycles. With a median follow up of 8 months (0.2-38), median progression-free survival was 5.5m, and median OS not reached (1y OS 81%).
Conclusion: In this phase I study exploring the combination of R+BV, the regimen was well tolerated and efficacious in MF/SS including heavily pretreated pts with advanced disease and prior exposure to HDACi and BV. Correlative studies, including analysis of changes in CD30 expression after 1 dose of R, and association of response with CD30 expression, are pending.
Disclosures
Barta:Affimed: Consultancy; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria; Seagen: Honoraria; Kyowa Kirin: Consultancy, Honoraria; Daiichi Sankyo: Consultancy. DeSimone:Helsinn: Speakers Bureau; Regeneron: Consultancy.
OffLabel Disclosure:
Brentuximab in non-CD30 positive CTCL and as first line therapy in CTCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal